valosin-containing protein (VCP) は、全ての真核生物と古細菌などに存在するATPaseで、
- ユビキチン-プロテアソーム系(Ubiquitin-proteasome system, UPS)
- アポトーシス
- オートファゴソーム形成 etc.
様々なタンパク分解系に関与し、
タンパク複合体、オルガネラ、クロマチンなど、大きなタンパクの分解に関与すると言われています [1]。
VCPの遺伝子変異は非常に稀ですが、
- 骨パジェット病と前頭側頭型認知症を伴う遺伝性封入体筋炎(inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia, IBMPFD)
- 筋萎縮性側索硬化症 (Amyotrophic lateral sclerosis, ALS)
- ハンチントン病 (Huntington's disease, HD)
などで報告されています。
今回、アメリカ・ペンシルベニア大学のDr. Leeらの研究グループは、VCP変異で空胞変性とアルツハイマー病理を生じた症例を報告しました [2]。
Neurodegeneration in Alzheimer's disease dementia is associated with neurofibrillary tangles composed of aggregated tau protein. Darwich et al. describe an additional form of autosomal-dominant dementia with neurofibrillary tangles linked to a hypomorph mutation in valosin-containing protein (VCP). VCP was found to disaggregate pathologic tau, and the hypomorph mutation increased tau accumulation in cells and mice. These findings highlight the role of protein turnover in maintaining neuronal health and suggest that VCP may provide a therapeutic target for Alzheimer's disease. Science , this issue p. [eaay8826][1] ### INTRODUCTION Alzheimer’s disease (AD) is a fatal neurodegenerative disease in which progressive brain degeneration compromises cognitive function. AD neurodegeneration is tightly associated with abnormal neuronal inclusions called neurofibrillary tangles, which are composed of aggregated tau protein. The importance of tau protein in dementia is highlighted by various known autosomal-dominant mutations in MAPT (microtubule-associated protein tau), the gene that encodes for tau, that are associated with frontotemporal lobar degeneration with tau inclusions (FTLD-tau). Identifying additional disease-causing genes that affect tau accumulation, including genes involved in protein quality control and tau clearance, has the potential to reveal previously unknown mechanisms that maintain neuronal health. ### RATIONALE Two families were identified with autosomal-dominant dementia linked to a p.Asp395Gly mutation in VCP . Valosin-containing protein (VCP) is a AAA+ [adenosine triphosphatases (ATPases) associated with diverse cellular activities] protein and uses energy from adenosine 5′-triphosphate (ATP) hydrolysis to unfold substrates to assist the dismantling of macromolecular complexes. Other VCP mutations have been identified in a disease called multisystem proteinopathy (MSP), which is associated with neuronal TDP-43 [TAR DNA-binding protein 43] protein inclusions. The mechanisms by which p.Asp395Gly VCP leads to neurodegeneration are unknown. ### RESULTS Two kindred were identified with an autosomal-dominant inheritance pattern of frontotemporal degeneration linked to a p.Asp395Gly VCP mutation. We have named this disease vacuolar tauopathy because of the presence of neuronal vacuoles and tau aggregates. Tau aggregates were morphologically and biochemically similar to AD neurofibrillary tangles. Moreover, the presence of vacuoles and neurofibrillary tangles in the brain were inversely correlated. Degenerating brain regions such as the frontal neocortex exhibited tau aggregation, whereas nondegenerating brain regions such as the visual cortex exhibited vacuolization. To further characterize the p.Asp395Gly VCP mutation, we assessed recombinant VCP proteins for ATPase activity in an in vitro assay. This approach demonstrated that p.Asp395Gly VCP exhibited a partial loss of ATPase activity, in contrast with MSP mutations, which increase ATPase activity. Given that VCP unfolds protein substrates, we hypothesized that VCP may disaggregate pathologic tau aggregates. Indeed, VCP appeared to partially disaggregate pathologic tau aggregates derived from AD human brain tissues, and the p.Asp395Gly mutation impaired this activity. VCP activity against pathologic tau was energy (ATP) dependent and required polyubiquitination of the tau substrate. In addition, expression of p.Asp395Gly VCP in a cell culture model of tau aggregation was associated with enhanced accumulation of cellular tau aggregates. Last, we generated mice in which the p.Asp395Gly mutation was knocked in, which exhibited a minimal phenotype when unchallenged. However, upon initiating tau aggregation through microinjection of pathologic AD tau extracts into the mouse brain, mutant VCP mice showed an increase in tau accumulation compared with that of wild-type animals. ### CONCLUSION We describe a partial loss-of-function mutation in VCP that was associated with a neurodegenerative disease, which we named vacuolar tauopathy. VCP appeared to exhibit activity that promoted the disruption of tau aggregates in an energy- and polyubiquitin-dependent manner. Furthermore, the p.Asp395Gly VCP mutation enhanced tau aggregation in a cell culture model system and in a knock-in mouse model. These results highlight a potential role for protein disaggregation in the maintenance of neuronal health. Furthermore, our findings suggest that VCP may provide a potential therapeutic target in the development of future AD therapies. ![Figure][2] Vacuolar tauopathy and VCP function. Model of tau aggregation, highlighting different mechanisms that lead to neurodegeneration in AD and FTLD-tau. Tau is a soluble protein (green) that forms highly structured fibrils (purple) in AD. Tau aggregates are also a hallmark of FTLD-tau. Autosomal dominant FTLD-tau mutations in MAPT , the gene that encodes tau protein, can alter splicing or enhance tau protein aggregation, which results in the accumulation of insoluble tau inclusions in neurons and glia. VCP (blue) appears to exhibit disaggregase activity against pathologic tau. This activity is dependent on the availability of energy (ATP) and the presence of polyubiquitin on tau protein aggregates. Vacuolar tauopathy is an autosomal-dominant form of dementia linked to a p.Asp395Gly VCP mutation (orange-red). Neurodegeneration in vacuolar tauopathy was associated with tau aggregates of similar biochemical composition and morphology as those seen in Alzheimer’s disease. The accumulation of insoluble tau aggregates in vacuolar tauopathy appeared to be in part due to a partial loss of tau disaggregase function associated with the p.Asp395Gly VCP mutation. WT, wild-type. Neurodegeneration in Alzheimer’s disease (AD) is closely associated with the accumulation of pathologic tau aggregates in the form of neurofibrillary tangles. We found that a p.Asp395Gly mutation in VCP (valosin-containing protein) was associated with dementia characterized neuropathologically by neuronal vacuoles and neurofibrillary tangles. Moreover, VCP appeared to exhibit tau disaggregase activity in vitro, which was impaired by the p.Asp395Gly mutation. Additionally, intracerebral microinjection of pathologic tau led to increased tau aggregates in mice in which p.Asp395Gly VCP mice was knocked in, as compared with injected wild-type mice. These findings suggest that p.Asp395Gly VCP is an autosomal-dominant genetic mutation associated with neurofibrillary degeneration in part owing to reduced tau disaggregation, raising the possibility that VCP may represent a therapeutic target for the treatment of AD. [1]: /lookup/doi/10.1126/science.aay8826 [2]: pending:yes
VCPがタウ凝集体を分解する ー "vacuolar tauopathy"
著者らは、アメリカとギリシャで、常染色体優性遺伝の前頭側頭型認知症(Frontotemporal dementia, FTD)の2家系を同定し、
遺伝子解析(ターゲット解析、エクソーム解析、全ゲノム解析)を行い、
VCPの遺伝子変異(c.1184A>G, p.Asp395Gly, NM_007126.5))を同定した。
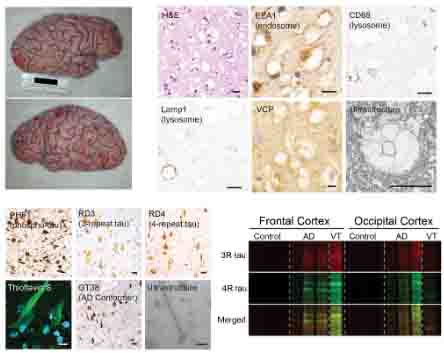
発端者の剖検を行ったところ、
マクロ所見では前頭葉に強い萎縮があり、皮質に空胞変性を認めた。
ミクロ所見では、神経細胞に空胞変性を認め、ニューロピルにVCPの顆粒状の染色性を認めた。
また、前頭葉、側頭葉、頭頂葉には、神経脱落を伴うタウ凝集体を認め、それらはアルツハイマー病(Alzheimer's disease (AD))病理で見られる神経原線維変化 (neurofibrillary tangles, NFTs) だった。
空胞変性とタウ病理の範囲は一致しておらず、
空胞変性は後頭葉優位、タウ変性は前頭葉優位だった。
アメリカの兄弟姉妹、およびギリシャ家系のMRI, タウPETなどを行ったところ、
発端者と同様に、後方優位の空胞変性と前方優位のタウ病理を認め、
これらの所見はVCP pAps395Gly変異に伴う所見と判断した。
in silico解析で、Asp395はVCPのタンパク分解機能に重要と判断し、
in vitroで、Asp395変異でVCPの活性が下がる事を確かめた。
VCPはタンパク凝集体の分解への関与が報告されており、
剖検脳ではNFTsにVCPの顆粒状の染色性を認めた事から、
VCPはタウ凝集体の分解に関与しているのではないかと考えた。
ADから抽出したタウ凝集体をリコンビナントのVCPと混合してチオフラビンアッセイを行ったところ、ATP依存性にタウ凝集体の分解を生じ、
p.Asp395Gly変異ではこの機能が阻害された。
同様の結果は、Fluorescence Resonance Energy Transfer (FRET) を用いた細胞系でも確認した。
これらの結果をin vivoでも確認するため、
著者らは、CRISPR-Cas9システムを用いて、p.Asp395Glyノックインマウスを作成した。
このマウス(3ヶ月齢)にAD脳から抽出したタウ凝集体を接種すると、
野生型マウスと比べて明らかなタウ病理の増悪を認めた。
以上の結果から、VCPはタウ凝集体の分解に関与し、p.Asp395Gly変異でその機能が障害され、家族性FTLDを発症すると考えられた。
My View
この仕事については、噂は色々聞いていたのですが、
コレスポのLee氏が「内密に事を進めたい」と言っていて、
合同カンファでも投稿直前まで全容を話してもらえませんでした。
合同カンファで、うちのPIは最後まで懐疑的な発言を繰り返していましたが、
私は、堅実な実験系で固められていて、素晴らしい内容だと思います。
今まで、タウの凝集に関与する遺伝子変異は報告されていましたが、
タウ凝集体の分解に関与する遺伝子変異の報告はなかったので、
これが初ということになります。
ただ、VCP変異と空胞変性との関連についてはまだよくわからないそうです。
タウ病理の分布と空胞変性の分布が異なる事から、別のメカニズムが働いているんじゃないかと思うのですが…
今後明らかになれば面白いと思います。
References
- Bo Kyoung Yeo & Seong-Woon Yu (2016) Valosin-containing protein (VCP): structure, functions, and implications in neurodegenerative diseases, Animal Cells and Systems, 20:6, 303-309, DOI: 10.1080/19768354.2016.1259181
- Darwich NF, Phan JM, Kim B, Suh E, Papatriantafyllou JD, Changolkar L, Nguyen AT, O'Rourke CM, He Z, Porta S, Gibbons GS, Luk KC, Papageorgiou SG, Grossman M, Massimo L, Irwin DJ, McMillan CT, Nasrallah IM, Toro C, Aguirre GK, Van Deerlin VM, Lee EB. Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau. Science. 2020 Oct 1:eaay8826. doi: 10.1126/science.aay8826. Epub ahead of print. PMID:33004675.



私もこのタウ凝集体形成と、神経毒性はきっと別じゃないかなと思いました。VCP KI mouseと、mTau KOを掛け合わせて、症状が悪化してしまうケースじゃないかなと思います。しかし、出来ているタウ線維は綺麗なPHFですね。
U PennでLee博士と言ったら、と思っていましたが、Edward Leeという方がいらっしゃるんですね。
そうですね。実は最初この話を聞いたとき、AD病理はCommonなのでただ合併しているだけじゃ…と思いましたが、内容を聞いて納得しました。
彼はEddieと呼ばれていて、大学生と院生の時期にうちのラボに在籍していたそうです。かなり博識で、とても話が面白いです。PIと親戚というわけではなさそうですw