今週月曜日、PfizerとBioNTechから、COVID19に対する新規ワクチンの効果についてのアナウンスメントがありました。[1, 2]
このワクチン、現在Phase 3が進行中ですが、現時点で90%の予防効果があるそう。
COVID19ワクチン(BNT162b2)の情報 [1, 3]
- SARS-CoV-2に対するmRNA-basedワクチン
- 治験参加者は43,538人 (38%はUS国民、42%は全世界…って、20%はどこ?)
- 参加者はワクチンとプラセボの2群に分けられ、21日の間隔を開けて2回接種
- 現時点で大きな副作用は報告されていない
- 94人がCOVID19にかかり、ワクチンの予防効果は90%以上
- ワクチンの効果がどれくらい長く持続するのかは不明だけれど、少なくとも1ヶ月以上
- 治験は、COVID19罹患者がエンドポイントの164人に達するまで継続される
Efficacyが50%でFDAの承認が得られるようなので [4]、
90%というのはかなり期待できる数字だと思います。
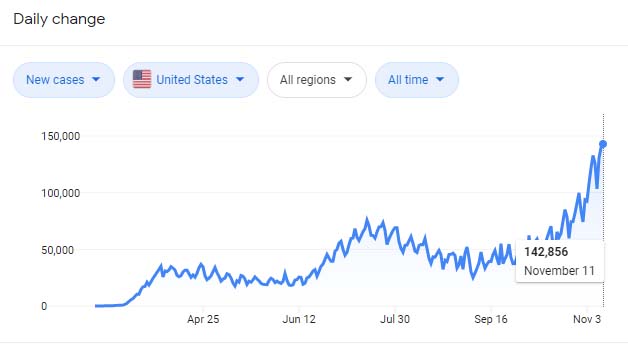
現在、アメリカのCOVID19新規感染者はうなぎのぼりで
子どもたちの通う小学校でも、
「来週から、学校の再度閉鎖を検討中」
というアナウンスメントがありました。
みんなが待ち望んでいるワクチン。
無事にFDAの承認がおりて、
できるだけ早く人々の元に届くといいな、と思います。

References
Vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis Analysis evaluated 94 confirmed cases of COVID-19 in trial participants Study enrolled 43,538 participants, with 42% having diverse backgrounds, and no serious safety concerns have been observed; Safety and additional efficacy data continue to be collected Submission for Emergency Use Authorization (EUA) to the U.S. Food and Drug Administration (FDA) planned for soon after the required safety milestone is achieved, which is currently expected to occur in the third week of November Clinical trial to continue through to final analysis at 164 confirmed cases in order to collect further data and characterize the vaccine candidate’s performance against other study endpoints Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2
The companies said an early analysis of results showed the vaccine to be more than 90% effective, beating expectations.
Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals - Full Text View.
Coronavirus vaccine developers now have some advice from the U.S. FDA. To win an approval, any vaccine must be at least 50% more effective than placebo in preventing the disease, the WSJ reports, citing guidance to be presented today. That's about as effective as a flu shot in a good year—but it falls short of some expert recommendations for arresting the virus' spread.