γセクレターゼは、アミロイド前駆タンパク(amyloid protein precuror, APP)を切断し、アミロイドβ (amyloid beta, Aβ)産生に関与する。
γセクレターゼの機能を調節する分子機構はいくつかあるが、Notchシグナリング等、APP以外のγセクレターゼ機能にも影響してしまうため、アルツハイマー病(Alzheimer's disease, AD)への臨床応用は難しかった。
韓ソウル国際大学のJungらの研究グループは、genome-wide functional スクリーニングを行い、γセクレターゼ活性を調節するタンパクを同定した。
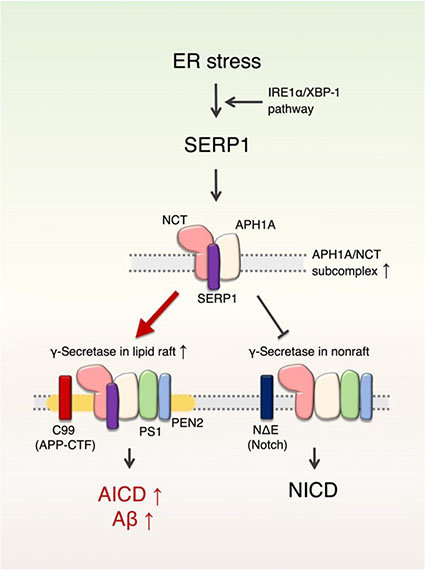
SERP1 (Stress Associated Endoplasmic Reticulum Protein 1) は、γセクレターゼのサブコンプレックス、APH1A/NCTに結合し、この集簇を高めてγセクレターゼ複合体の活性を上げた。
SERP1は、小胞体(endoplasmic reticulum, ER)ストレスに反応して、γセクレターゼ複合体をリピッドラフトに移動させてAPPに作用し、Aβ産生量を増加させていた。
この動きにより、SERP1は、Notchにはほとんど影響を及ぼさなかった。
細胞を高血糖下に置いたり、3xTg ADマウスにSTZをi.p.して糖尿病にさせると、SERP1量、γセクレターゼ活性、Aβ産生量が増加した。
逆に、マウスの海馬でSERP1をノックダウンすると、Aβ産生量は減少した。
SERP1は、APP(のみ)に働きかけるγセクレターゼ調節分子といえ、ERストレスの関与する糖尿病やADに治療ターゲットとして期待できる。
The accumulation of β-amyloid (Aβ) plaques in the brain contributes to the symptoms of Alzheimer’s disease (AD). Aβ is generated by the γ-secretase–mediated cleavage of amyloid precursor protein (APP), and γ-secretase activity is induced by cell stress, such as occurs with obesity and diabetes, which are major risk factors for AD. However, the use of γ-secretase inhibitors is limited by their disruption of other γ-secretase substrates. Using a gain-of-function screen in cells, diabetic AD model mice, and postmortem samples from patients with AD, Jung et al. identified the protein SERP1 as a cell stress–induced, APP-biased activator of γ-secretase. Knocking down SERP1 in cells or the mouse hippocampus decreased Aβ production, suggesting that blocking SERP1 might be a more selective therapeutic to reduce Aβ plaque formation in patients with AD. The enzyme γ-secretase generates β-amyloid (Aβ) peptides by cleaving amyloid protein precursor (APP); the aggregation of these peptides is associated with Alzheimer’s disease (AD). Despite the development of various γ-secretase regulators, their clinical use is limited by coincident disruption of other γ-secretase–regulated substrates, such as Notch. Using a genome-wide functional screen of γ-secretase activity in cells and a complementary DNA expression library, we found that SERP1 is a previously unknown γ-secretase activator that stimulates Aβ generation in cells experiencing endoplasmic reticulum (ER) stress, such as is seen with diabetes. SERP1 interacted with a subcomplex of γ-secretase (APH1A/NCT) through its carboxyl terminus to enhance the assembly and, consequently, the activity of the γ-secretase holoenzyme complex. In response to ER stress, SERP1 preferentially recruited APP rather than Notch into the γ-secretase complex and enhanced the subcellular localization of the complex into lipid rafts, increasing Aβ production. Moreover, SERP1 abundance, γ-secretase assembly, and Aβ production were increased both in cells exposed to high amounts of glucose and in diabetic AD model mice. Conversely, Aβ production was decreased by knocking down SERP1 in cells or in the hippocampi of mice. Compared to postmortem samples from control individuals, those from patients with AD showed increased SERP1 expression in the hippocampus and parietal lobe. Together, our findings suggest that SERP1 is an APP-biased regulator of γ-secretase function in the context of cell stress, providing a possible molecular explanation for the link between diabetes and sporadic AD.